United Arab Emirates (UAE) is now doing clinical trials for Coronavirus Disease 2019 (COVID-19) vaccine, and an Ilonggo nurse working there has volunteered to be part of it.
Ramon Francisco Sonico, a native of Sta. Barbara, Iloilo, said that he first heard of the clinical trials of UAE government and Chinese company Sinopharm in social media. He registered in the program, and two weeks later, he was notified that he is approved to take part in the clinical trials.
“I have full trust and confidence with the UAE government. They won’t risk for this kind of trial if they knew it will give harm to people in this country,” Sonico said, in an interview over GMA News. “It’s all free; the cost will be shouldered by the company (Sinopharm) and the UAE government.”
Sonico, who finished his nursing degree at Central Philippine University, said that on Sunday he got his first shot of inactivated SARS-CoV-2 vaccine (vero cell) developed by Sinopharm and was approved by DOH to carry out clinical trials in accordance to UAE regulations.
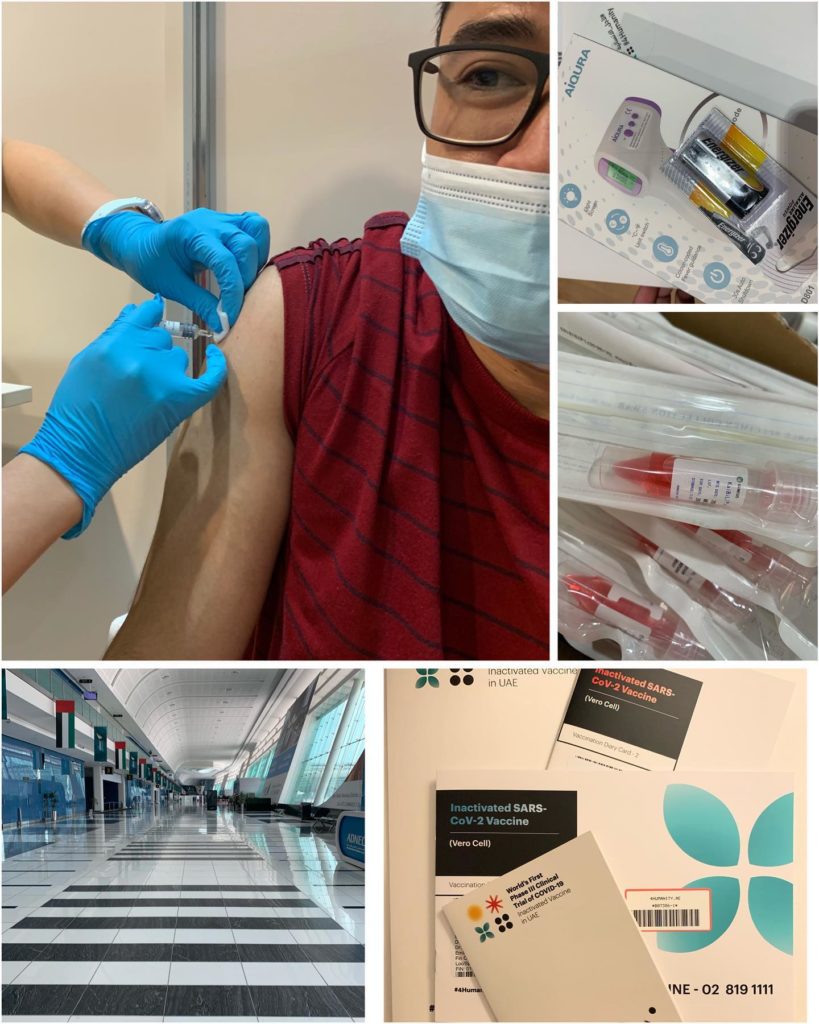
“UAE is very confident to find solutions to fight COVID. The flow was smooth from registrations (full medical work out and informed consent) to vital signs checking to blood extractions for antibodies to COVID swab and finally vaccine injection,” Sonico posted on his Facebook account.
“After vaccination, I stayed another 30 minutes for observations and they provided me food and freebies. Follow up checkup by means of telemedicine will be done the next day,” Sonico added. “So far I feel completely fine. Let’s hope this trial will make a positive result and make the world COVID-free. All for humanity.”
UAE began the first globally-recognized, last-phase (Phase 3) clinical trial for a COVID-19 vaccine in Abu Dhabi on July 16. This is the final step before a vaccine is approved for use among the public.
The Phase 3 clinical trial is being led by Abu Dhabi’s health department in partnership with Group 42 and Chinese drug maker Sinopharm.
Nearly 5,000 people living in the Abu Dhabi and Al Ain signed up in the first 24 hours, and up to 15,000 volunteers who are in good health and between the ages of 18 to 60, are needed in the trials. (With reports from GMA News and The National)




